Retinal Vein Occlusions (RVO)
What is a retinal vein occlusion?
Blood flows through our body in blood vessels. Each organ in your body receives nutrients and oxygen via an artery and excretes waste products and carbon dioxide through a vein. The eye is no exception. Vein occlusion is a condition whereby the retinal vein is blocked in the eye and as a result the retina becomes engorged in blood and fluid (oedema, Fig 1a and 1b). Visual blurring can range from being asymptomatic and mild to complete blindness depending on severity of the condition. This condition is of sudden onset and it tends to be painless. Several risk factors exist for developing retinal vein occlusion. Most common risk factors include diabetes, hypertension, raised eye pressure and increased blood viscosity.
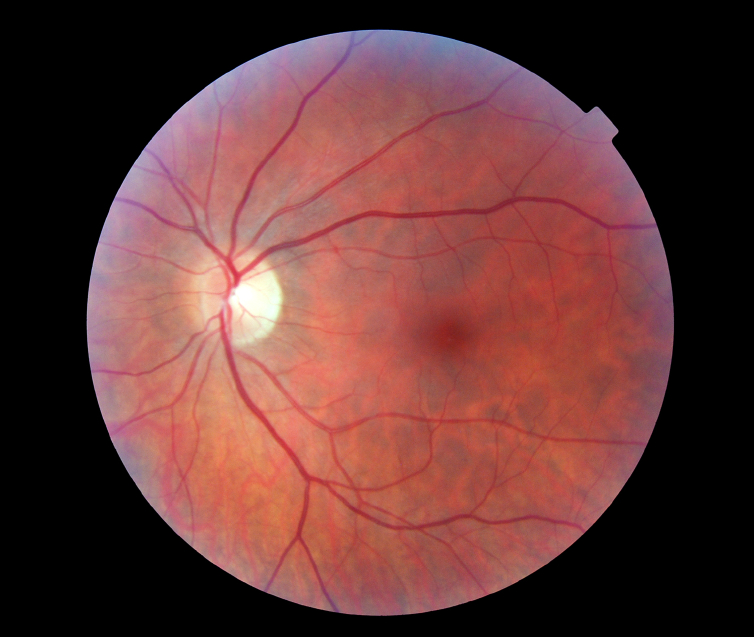
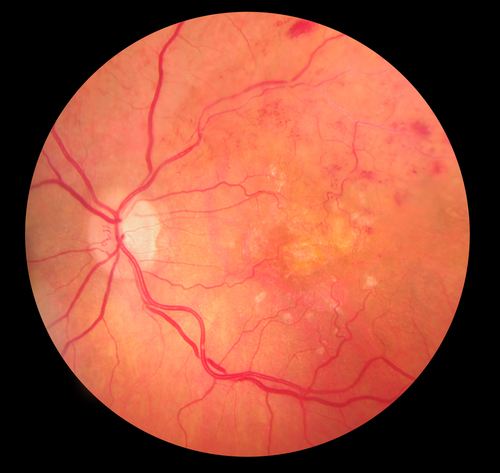
What are the different types and symptoms of RVO?
What are the different treatment options?
What should I expect after an injection?
Take home message is if in doubt please call in and get your symptom checked.
What are the risks with injection treatment?
Major risks (uncommon)
- Serious eye infection (1 in every 1000 injections)
- Very high pressure in the eye
- Tear or detachment in the retina
- Traumatic cataract
- Blood clots or bleeding inside the eye
- Inflammation in the eye
- Potential for heart attack and/or stroke – only theoretical
Minor risks (common)
- Red eye (bleed at the superficial surface of the eye)
- Sore gritty eye (usually first 48 hours post injection)
- Small specks (floaters ) or transient flashing lights may be seen in your vision for few days
Can I have cataract surgery if I have retinal vein occlusion?
What is the difference between anti VEGF injection like Lucentis and an Ozurdex implant?
What are the advantages of private treatment?
Private companies will fund a certain part of your treatment depending on your level of cover. The advantage would include having a named surgeon deliver your treatment at a time and place convenient to you. The choice of agent, duration of treatment and the financial implications (in case of self funding patients) will be discussed in full at time of consultation.
NICE website guidance links
https://www.nice.org.uk/guidance/TA283 (Ranibizumab)
https://www.nice.org.uk/guidance/TA305 (Aflibercept)
Aflibercept for treating visual impairment caused by macular oedema secondary to central retinal vein occlusion
NICE technology appraisal guidance [TA305] Published date: 26 February 2014